Formula For Enthalpy Of Vaporization
The enthalpy of vaporization, (symbol ∆Hvap; unit of measurement: J) or heat of evaporation is the amount of energy required to change phase from liquid to gas phase. Thermal Engineering
Enthalpy in Intensive Units – Specific Enthalpy
The enthalpy tin can be made into an intensive, or specific, variable by dividing past the mass. Engineers utilise the specific enthalpy in thermodynamic analysis more than the enthalpy itself. The specific enthalpy (h) of a substance is its enthalpy per unit mass. It equals to the total enthalpy (H) divided by the full mass (m).
h = H/m
where:
h = specific enthalpy (J/kg)
H = enthalpy (J)
m = mass (kg)
Annotation that the enthalpy is the thermodynamic quantity equivalent to the full heat content of a organisation. The specific enthalpy is equal to the specific internal energy of the system plus the product of pressure and specific volume.
h = u + pv
In general, enthalpy is a belongings of a substance, like pressure, temperature, and volume, simply it cannot be measured directly. Normally, the enthalpy of a substance is given with respect to some reference value. For example, the specific enthalpy of water or steam is given using the reference that the specific enthalpy of water is zero at 0.01°C and normal atmospheric pressure, where hL = 0.00 kJ/kg. The fact that the absolute value of specific enthalpy is unknown is non a problem, still, because it is the change in specific enthalpy (∆h) and not the absolute value that is important in practical bug.
See also: Steam Tables
Energy Balance in a Steam Generator
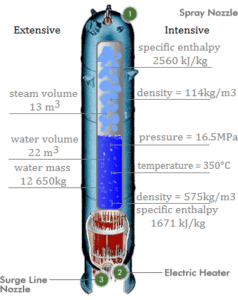
Calculate the corporeality of main coolant, which is required to evaporate 1 kg of feedwater in a typical steam generator. Assume that there are no energy losses, this is only idealized instance.
Remainder of the master excursion
The hot primary coolant (water 330°C; 626°F; 16MPa) is pumped into the steam generator through primary inlet. The chief coolant leaves (water 295°C; 563°F; 16MPa) the steam generator through primary outlet.
hI, inlet = 1516 kJ/kg
=> ΔhI = -206 kJ/kg
hI, outlet = 1310 kJ/kg
Balance of the feedwater
The feedwater (water 230°C; 446°F; 6,5MPa) is pumped into the steam generator through the feedwater inlet. The feedwater (secondary excursion) is heated from ~230°C 446°F to the boiling point of that fluid (280°C; 536°F; 6,5MPa). Feedwater is then evaporated and the pressurized steam (saturated steam 280°C; 536°F; 6,5 MPa) leaves the steam generator through steam outlet and continues to the steam turbine.
h2, inlet = 991 kJ/kg
=> ΔhII = 1789 kJ/kg
hTwo, outlet = 2780 kJ/kg
Balance of the steam generator
Since the difference in specific enthalpies is less for primary coolant than for feedwater, information technology is obvious that the amount of primary coolant volition be higher than 1kg. To produce of i kg of saturated steam from feedwater, nigh 1789/206 x ane kg = viii.68 kg of primary coolant is required.
Enthalpy of Vaporization

In full general, when a textile changes stage from solid to liquid, or from liquid to gas a certain amount of energy is involved in this change of phase. In case of liquid to gas stage change, this amount of free energy is known as the enthalpy of vaporization, (symbol ∆Hvap; unit of measurement: J) as well known as the (latent) oestrus of vaporization or heat of evaporation. Latent heat is the amount of heat added to or removed from a substance to produce a alter in phase. This energy breaks downwardly the intermolecular attractive forces, and likewise must provide the energy necessary to expand the gas (the pΔV piece of work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
Latent heat of vaporization – h2o at 0.i MPa (atmospheric pressure)
hlg = 2257 kJ/kg
Latent heat of vaporization – water at iii MPa (pressure within a steam generator)
hlg = 1795 kJ/kg
Latent heat of vaporization – water at 16 MPa (pressure inside a pressurizer)
hlg = 931 kJ/kg
The heat of vaporization diminishes with increasing pressure, while the boiling point increases. It vanishes completely at a certain point called the critical betoken. Above the critical signal, the liquid and vapor phases are indistinguishable, and the substance is called a supercritical fluid.
The heat of vaporization is the heat required to completely vaporize a unit of saturated liquid (or condense a unit of measurement mass of saturated vapor) and it equal to hlg = hg − hl .
The heat that is necessary to melt (or freeze) a unit of measurement mass at the substance at constant pressure level is the heat of fusion and is equal to hsl = hl − hs , where hs is the enthalpy of saturated solid and hl is the enthalpy of saturated liquid.

Specific Enthalpy of Wet Steam
 The specific enthalpy of saturated liquid water (x=0) and dry steam (ten=1) can be picked from steam tables. In case of moisture steam, the actual enthalpy can exist calculated with the vapor quality, x, and the specific enthalpies of saturated liquid water and dry out steam:
The specific enthalpy of saturated liquid water (x=0) and dry steam (ten=1) can be picked from steam tables. In case of moisture steam, the actual enthalpy can exist calculated with the vapor quality, x, and the specific enthalpies of saturated liquid water and dry out steam:
h wet = h south ten + (one – x ) h l
where
h moisture = enthalpy of wet steam (J/kg)
h south = enthalpy of "dry" steam (J/kg)
h l = enthalpy of saturated liquid h2o (J/kg)
Equally can exist seen, moisture steam will ever have lower enthalpy than dry steam.
Example:
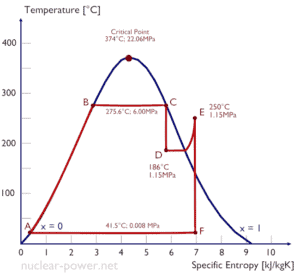
A high-force per unit area phase of steam turbine operates at steady state with inlet conditions of 6 MPa, t = 275.vi°C, x = ane (point C). Steam leaves this stage of turbine at a pressure of 1.15 MPa, 186°C and x = 0.87 (point D). Calculate the enthalpy departure between these two states.
The enthalpy for the state C tin exist picked straight from steam tables, whereas the enthalpy for the state D must be calculated using vapor quality:
h i, moisture = 2785 kJ/kg
h two, wet = h 2,s x + (i – x ) h ii,l = 2782 . 0.87 + (1 – 0.87) . 790 = 2420 + 103 = 2523 kJ/kg
Δh = 262 kJ/kg
References:
Reactor Physics and Thermal Hydraulics:
- J. R. Lamarsh, Introduction to Nuclear Reactor Theory, second ed., Addison-Wesley, Reading, MA (1983).
- J. R. Lamarsh, A. J. Baratta, Introduction to Nuclear Applied science, 3d ed., Prentice-Hall, 2001, ISBN: 0-201-82498-1.
- Westward. M. Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-i.
- Glasstone, Sesonske. Nuclear Reactor Engineering: Reactor Systems Engineering, Springer; 4th edition, 1994, ISBN: 978-0412985317
- Todreas Neil E., Kazimi Mujid South. Nuclear Systems Volume I: Thermal Hydraulic Fundamentals, 2d Edition. CRC Press; 2 edition, 2012, ISBN: 978-0415802871
- Zohuri B., McDaniel P. Thermodynamics in Nuclear Power Found Systems. Springer; 2015, ISBN: 978-iii-319-13419-2
- Moran Michal J., Shapiro Howard N. Fundamentals of Engineering Thermodynamics, Fifth Edition, John Wiley & Sons, 2006, ISBN: 978-0-470-03037-0
- Kleinstreuer C. Modern Fluid Dynamics. Springer, 2010, ISBN 978-1-4020-8670-0.
- U.South. Department of Energy, THERMODYNAMICS, Heat TRANSFER, AND FLUID FLOW. DOE Fundamentals Handbook, Volume ane, 2 and 3. June 1992.
Nosotros promise, this article, Enthalpy of Vaporization, helps you. If and then, give us a like in the sidebar. Main purpose of this website is to assist the public to acquire some interesting and important information well-nigh thermal engineering science.
Formula For Enthalpy Of Vaporization,
Source: https://www.thermal-engineering.org/what-is-enthalpy-of-vaporization-definition/
Posted by: coxource1977.blogspot.com


0 Response to "Formula For Enthalpy Of Vaporization"
Post a Comment